Antidepressants and Psychedelics
Psychedelics may help depression — but are they safe to use with SSRIs?
Written by Camile Bahi for the MIND Blog.

Disclaimer: Before altering their antidepressant medication schemes, patients should always consult with their general physician or psychiatrist in order to prevent withdrawal syndrome and eventual relapse.
Most recent clinical trials with psychedelics either asked participants to stop taking their antidepressant medications before enrolling, or they rejected patients that were taking antidepressants. Similarly, some psychedelic retreat centers only allow participants who are not currently taking antidepressants.
As psychedelics have shown initial promise for hard-to-treat depression, an often-raised concern relates to the possible risks of the interaction between antidepressant drugs and psychedelics. This article aims to investigate this issue and provide some preliminary answers.
Classical psychedelics are defined by their ability to act as serotonin receptor agonists, particularly at the Serotonin 2A receptor (5HT2AR). Accordingly, many of the subjective and biological effects of classical psychedelics are blocked after administering 5HT2AR antagonists such as ketanserin. Classical psychedelics have been shown to be relatively safe in the context of clinical trials, with notable side effects being mild headaches, small elevations in blood pressure, and acute anxiety, none of which commonly require medical intervention. Although the exact mechanisms of action and the pharmacokinetics of these molecules are not completely understood, two particular issues have been raised when it comes to their interaction with antidepressant drugs: the so-called serotonin syndrome and a decrease in subjective psychedelic effects.
Serotonin Syndrome: Antidepressants and Psychedelics
Serotonin syndrome is a potentially lethal adverse drug reaction that is most likely to occur when two compounds able to raise serotonin neurotransmission are taken simultaneously. However, it is also known to occur after only one such compound. Serotonin is produced from the amino acid L-tryptophan and its effects are regulated by re-uptake mechanisms, feedback loops, and enzymes such as monoamine oxidase. Serotonin acts on the nervous system both at the central and at the peripheral level. In the central nervous system, it plays a role in awareness, behavior, muscle tone, body temperature, and pain. In the periphery, it regulates vascular tone, nociception (pain perception), and gastric activity. The effects of serotonin are mediated through 7 receptors types (from 5-HT1 to 5-HT7) and at least 14 subtypes.
Serotonin syndrome has been reported in all age groups and is estimated to occur in approximately 15% of persons who overdose on SSRIs. Nevertheless, an accurate incidence rate of this syndrome is difficult to obtain because its symptoms lack specificity, and because of the lack of awareness concerning this condition among physicians. A survey found that 85% of physicians were unaware of the existence of this toxidrome.
The severity of serotonin syndrome can vary from mild to life-threatening. Its symptoms are often described as a clinical triad: neuromuscular abnormalities (such as tremor or muscle hypertonicity leading to hyperthermia), autonomic nervous system hyperactivity (leading to increased heart rate and diarrhea), and changes in mental state (such as agitation or delirium; see figure 1 for an overview).
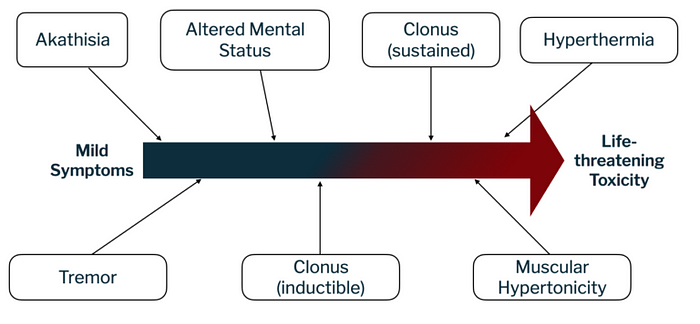
The precise pathophysiology of serotonin syndrome is not completely understood, but it appears to result from an excess in serotonin neurotransmission. It is not linked to a single serotonin receptor, even though it is suggested that 5-HT2A receptors substantially contribute to the condition. Other receptors, such as 5-HT1A, appear to also contribute to it when serotonin concentrations reach a point where all other receptor subtypes are saturated (see figure 2 for a visualization).Other neurotransmitters, such as norepinephrine, may also play an important role in this syndrome: for example, an increase in CNS norepinephrine concentration during serotonin syndrome correlates with more severe symptoms. Dopamine could also be involved, since an increase in dopamine neurotransmission can indirectly trigger serotonin release.
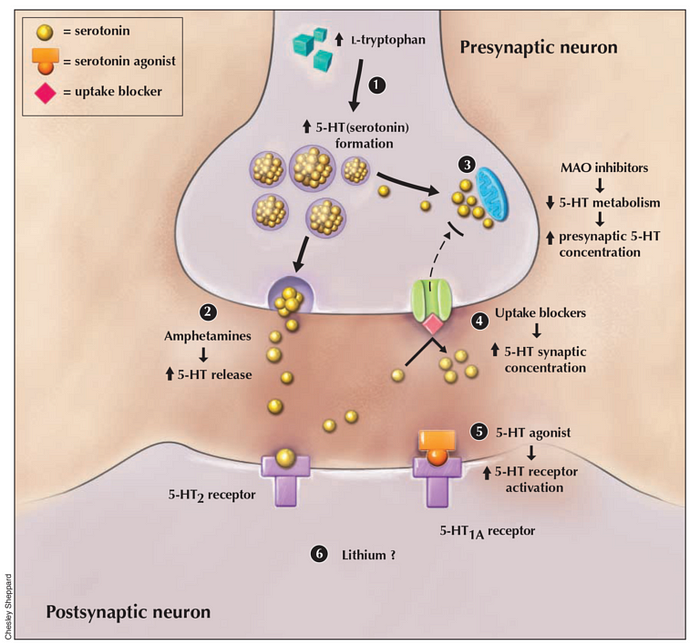
In most countries, prescribing more than one serotonin medication at once is contraindicated, since this can lead to serotonin syndrome. As detailed in Table 1 below, every medication that increases serotonin neurotransmission can be involved in its pathogenesis. Most cases involve SSRI or monoamine oxidase inhibitor (MAOI) drugs, taken simultaneously with at least one other medication raising serotonin levels. Combining drugs that differentially increase serotonin neurotransmission is more likely to induce severe serotonin syndrome.

There is no empirical data on the interaction between psychedelics and antidepressants, and whether this raises the risk of serotonin syndrome. Nevertheless, it is well-known that psychedelics have 5-HT2AR agonist properties, therefore increasing 5-HT2AR neurotransmission. Thus, from a pharmacological point of view, it seems likely that their co-administration with serotonergic antidepressants could induce serotonin syndrome. Accordingly, it could be dangerous to mix psychedelics with any type of medication that increases serotonergic neurotransmission.
Furthermore, it is important to note that several psychedelics, such as LSD and 5-MeO-DMT, are metabolized by CYP2D6, a liver enzyme that is involved in metabolizing many substances. At the same time, SSRIs are both a substrate and an inhibitor of this enzyme. This means that CYP2D6 is less available for metabolizing both psychedelics and SSRIs, resulting in an increase in blood concentrations of serotonergic substances, which is associated with the induction of serotonin syndrome.
For this reason, it is recommended to taper antidepressant medication before engaging in any psychedelic therapy or usage. Moreover, since administration of serotonin agents within five weeks after the discontinuation of SSRIs has been shown to induce serotonin syndrome, it appears to be safer to wait for at least five weeks before using any psychedelic compound.
Antidepressant Drugs Blunting Subjective Psychedelic Effects
In addition to the risk of serotonin syndrome, the subjective effects of the psychedelic experience may be altered if psychedelics are combined with antidepressants. Anecdotal evidence suggests that the acute use of SSRIs, as well as chronic use of tricyclic antidepressants, can alter the subjective effects of psychedelics. Regarding tricyclic antidepressants, this observation could be related to postsynaptic receptor sensitization and increased dopamine levels, which indirectly lead to an increase in serotonin neurotransmission.
Conversely, chronic administration of SSRIs or MAOIs have been shown to decrease the subjective effects of psychedelics. Concerning SSRIs, a possible reason for this could be that the chronic administration of SSRIs causes downregulation of 5-HT2AR receptors. This, in turn, may make someone less sensitive to substances affecting these receptors, such as psychedelics. Therefore, this decrease in subjective effects may result from 5-HT2AR downregulation. Concerning MAOIs, chronic administration has been shown to trigger serotonin receptor desensitization, which could explain the observed decrease in subjective psychedelic effects.
The precise mechanisms underlying the modulation of the psychedelic experience by antidepressants are still unclear, and further research should be conducted in order to gain a better understanding. Moreover, the risk of serotonin syndrome cannot be ignored due to the observation of blunted subjective effects of psychedelics following 5-HT2AR down-regulation. Specifically, it might be the case that serotonin syndrome depends on the rate of occupied 5-HT receptors, whereby a higher rate of occupied receptors could be reached more easily when the global number of receptors is lowered. Moreover, several inter-individual variations, such as genetic variations in the enzymes responsible for drug metabolism, could also play a role in the occurrence of serotonin syndrome.
Some Concluding Thoughts
It is impossible to state with certainty what the relationship is between serotonin syndrome and the pharmacology of psychedelics. Current knowledge is insufficient for formulating an accurate assessment of the risk, or a model of the modulation of both subjective and physiological effects arising from the combination of antidepressants and psychedelic substances.
There has not been much progress in elucidating the pathophysiology of serotonin syndrome in recent years. However, since we are gaining a clearer view of the pharmacology of psychedelics, we might develop more precise recommendations for clinical work in the future.
Nevertheless, a close look at the effects of common antidepressants and psychedelic drugs on serotonin neurotransmission suggests that this combination is risky from a pharmacological point of view, and that it would be very unlikely to have clinically beneficial effects. Consequently, caution is advised when considering taking a psychedelic while on antidepressant medication.

The MIND Foundation for Psychedelic Research aims to create a healthier, more connected world through research and education. Learn more or become part of our mission.
References appear in the original article.
